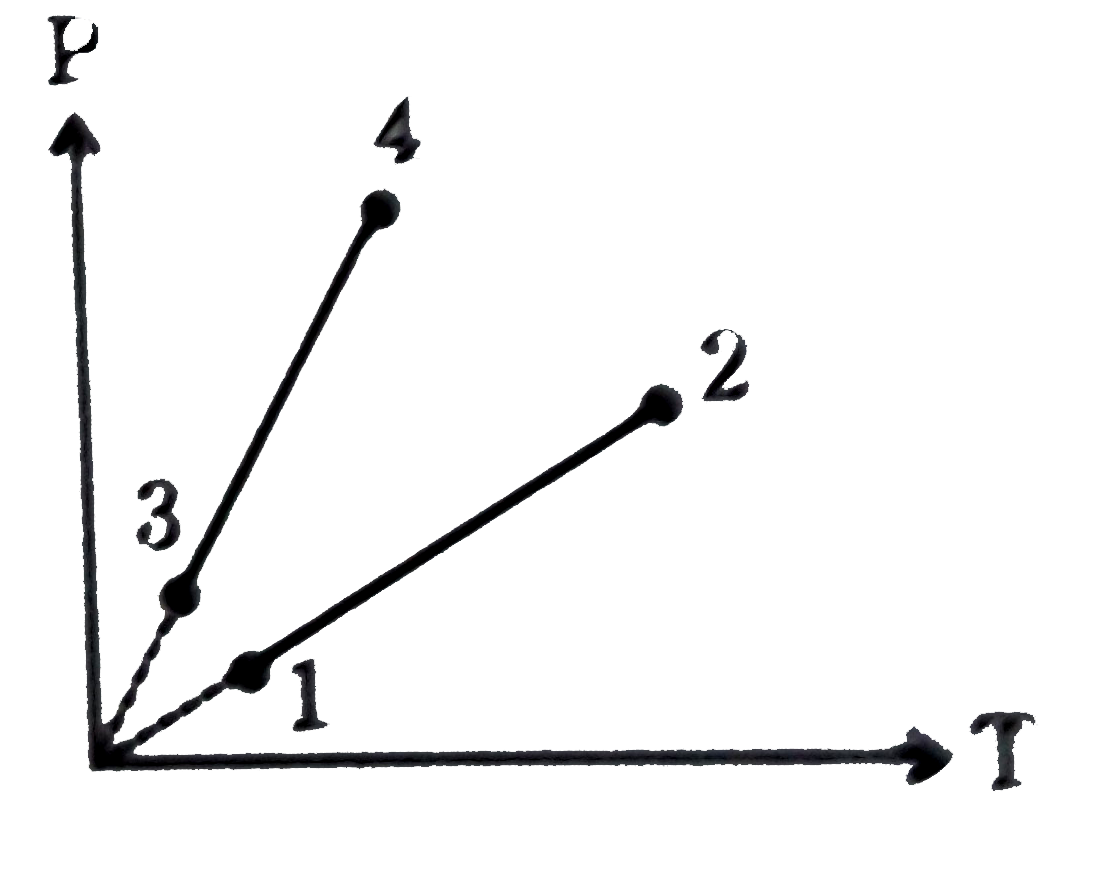
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Pressure versus temperature graph of an ideal gas of equal number of m...
Text Solution
|
- Pressure versus temperature graph of an ideal gas of equal number of m...
Text Solution
|
- Pressure versus temperature graph of an ideal gas as shown in Fig. Cor...
Text Solution
|
- Pressure versus temperature graphs of an ideal gas are as shown in fig...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- Pressure versus temperature graph of an ideal gas of equal number of m...
Text Solution
|
- A plot of volume (V) versus temperature (T) for a gas at constant pres...
Text Solution
|
- Pressure versus temperature graph of an ideal gas are as shown in figu...
Text Solution
|