A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two different isotherms representing the relationship between pressure...
Text Solution
|
- Two identical containers A and B having volume of ideal gas at the sam...
Text Solution
|
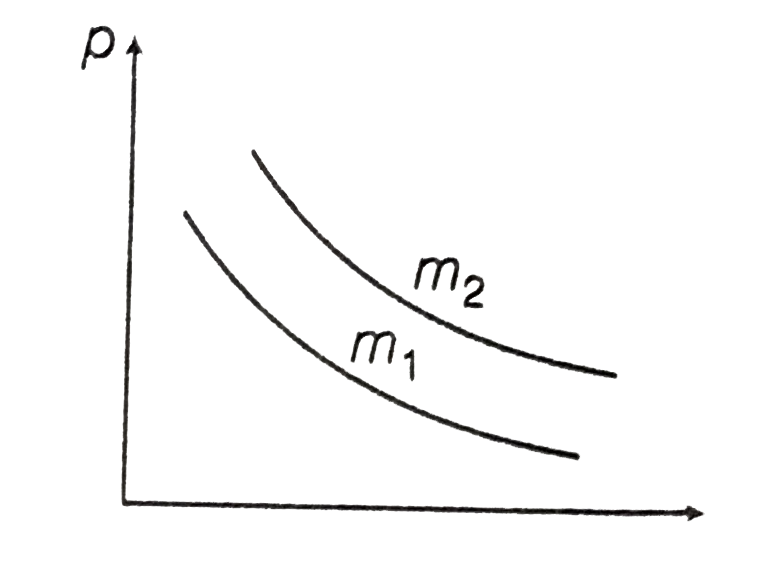
- The figure shown, the p-V diagram of two different masses m(1) and m(2...
Text Solution
|
- Two different isotherms representing the relationship between pressure...
Text Solution
|
- घर्षणहीन पिस्टनों वाले दो समरुप बेलनाकार बर्तनों A तथा B में एक ही ...
Text Solution
|
- Two identical vessels A and B with frictionless pistons contain the sa...
Text Solution
|
- For same mass of two different ideal gases of molecular weights M(1) a...
Text Solution
|
- For same mass of two different ideal gases of molecular weights M(1) a...
Text Solution
|
- Two identical containers A and B with frinctionless pistons contain th...
Text Solution
|