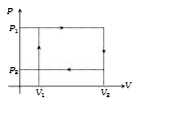
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- n moles of a Vander Waal's gas obeying the equation of state (P+(n^2a)...
Text Solution
|
- The vander Waal's equation for n moles of a real gas is (p + (a)/(V^(...
Text Solution
|
- The Van der waal's equation for n moles of a real gas is given by (P+(...
Text Solution
|
- A van der Waal's gas obeys the equation of state (P+(n^(2)a)/(V^(2)))(...
Text Solution
|
- The van der Waals, equation for n moles of real gas is: (P+(n^(2)a)/(...
Text Solution
|
- The vander waal's equation for a gas is (p + a/V^(2))(V - b) = nRT whe...
Text Solution
|
- A real gas obeying van der Waals' equation : ( P + ( an^(2))/( V^(2))...
Text Solution
|
- van der Waal's equation for a gas is stated as, P=(nRT)/(V-nb)-a(n/V)^...
Text Solution
|
- One mole of a van der Waals PA gas obeying the equation P (P + a/V^(2)...
Text Solution
|