A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
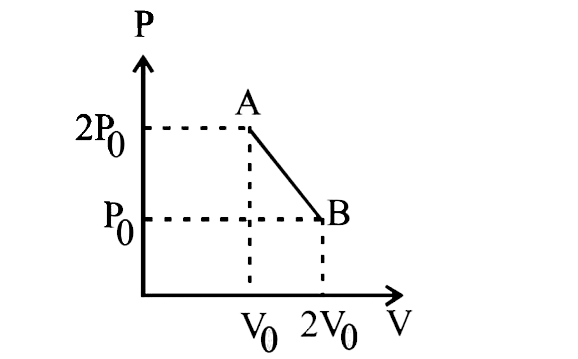
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- One mole of an ideal monotomic gas undergoes a linear process from A t...
Text Solution
|
- One mole of monoatomic ideal gas undergoes a process ABC as shown in f...
Text Solution
|
- n' moles of an ideal gas undergoes a process A to B as shown in the fi...
Text Solution
|
- Three moles of an ideal gas undergo a cyclic process shown in figure....
Text Solution
|
- n मोल आदर्श गैस एक प्रक्रम A to B से गुजरती है (चित्र देखिए) | इस प्र...
Text Solution
|
- n mol The ideal gas is shown in the figure AB Was taken through the pr...
Text Solution
|