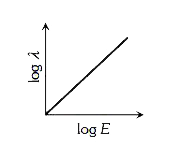
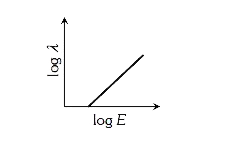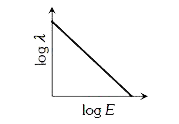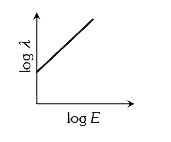A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The log - log graph between the energy E of an electron and its de - B...
Text Solution
|
- In an excited state of hydrogen like atom an electron has total energy...
Text Solution
|
- The log - log graph between the energy E of an electron and its de - B...
Text Solution
|
- The de Broglie wavelength (lambda) of a particle is related to its kin...
Text Solution
|
- An electron is in excited state in a hydrogen-like atom. It has a tota...
Text Solution
|
- किसी इलेक्ट्रॉन की ऊर्जा (E) तथा दी-ब्रोग्ली तरंगदैर्ध्य (lambda) के म...
Text Solution
|
- If E and lambda represent the energy and wavelenght respectively of an...
Text Solution
|
- Identify the graph depicting the variation of the de Broglie wavelengt...
Text Solution
|
- The graph between momentum p and de-Broglie wavelength lambda of photo...
Text Solution
|