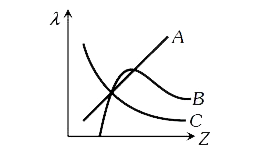
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The variation of wavelength lamda of the Kalpha line with atomic num...
Text Solution
|
- Kalpha wavelength emitted by an atom of atomic number Z=11 is lambda ....
Text Solution
|
- The variation of wavelength lambda of the K(alpha) line with atomic nu...
Text Solution
|
- In X-ray spectrum wavelength lamda of line K(alpha) depends on atomic ...
Text Solution
|
- The wavelength of kalpha line in the X-ray spectrum for tungsten (Z=74...
Text Solution
|
- If Z is the atomic number of the target atom, then the frequency of th...
Text Solution
|
- The two elements have their atomic numbers as Z1 and Z2 The ratio of t...
Text Solution
|
- The ratio of wavelength of Kalpha line of an element with atomic numbe...
Text Solution
|
- The wavelength of K(alpha) line from an element of atomic number 41 is...
Text Solution
|