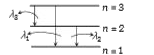
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Due to transitions among its first three energy levels, hydrogenic ato...
Text Solution
|
- The wavelength of the first Lyman lines of hydrogen He^(+) and Li^(2+)...
Text Solution
|
- Due to transitions among its first three energy levels, hydrogenic ato...
Text Solution
|
- If lambda(1), lamda(2)and lamda(3) are the wavelengths of the wave giv...
Text Solution
|
- Energy levels A,B and C of a certain atom correspond to increasing val...
Text Solution
|
- A dye absorbs a photon of wavelength lamda and re-emits the same energ...
Text Solution
|
- The matrix A={:[(lamda(1)^(2),lamda(1)lamda(2),lamda(1)lamda(3)),(lam...
Text Solution
|
- If lamda(1) and lamda(2) are the wavelengths of the first members of t...
Text Solution
|
- For Balmer series, wavelength of first line is lamda(1) and for Bracke...
Text Solution
|