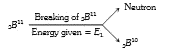A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The binding energy per nucleon of .(5)B^(10) is 8 MeV and that of .(5)...
Text Solution
|
- The binding energy per nucleon of O^16 is 7.97 MeV and that of O^17 is...
Text Solution
|
- The binding energy per nucleon of .(5)B^(10) is 8 MeV and that of .(5)...
Text Solution
|
- The binding energy per nucleon of .(5)B^(10) is 8.0 MeV and that of .(...
Text Solution
|
- A neutron breaks into a proton and electron. Calculate the energy prod...
Text Solution
|
- Calculate the ratio between the wavelength of an electron and a proton...
Text Solution
|
- Find the rest mass energy of a proton in MeV. [m(p) = 1.673 xx 10^(-27...
Text Solution
|
- The binding energy per nucleon for C^(12) is 7.68 MeV and that for C^(...
Text Solution
|
- If the binding energy of N^(14) is 7 . 5 Mev per nucleons and that...
Text Solution
|