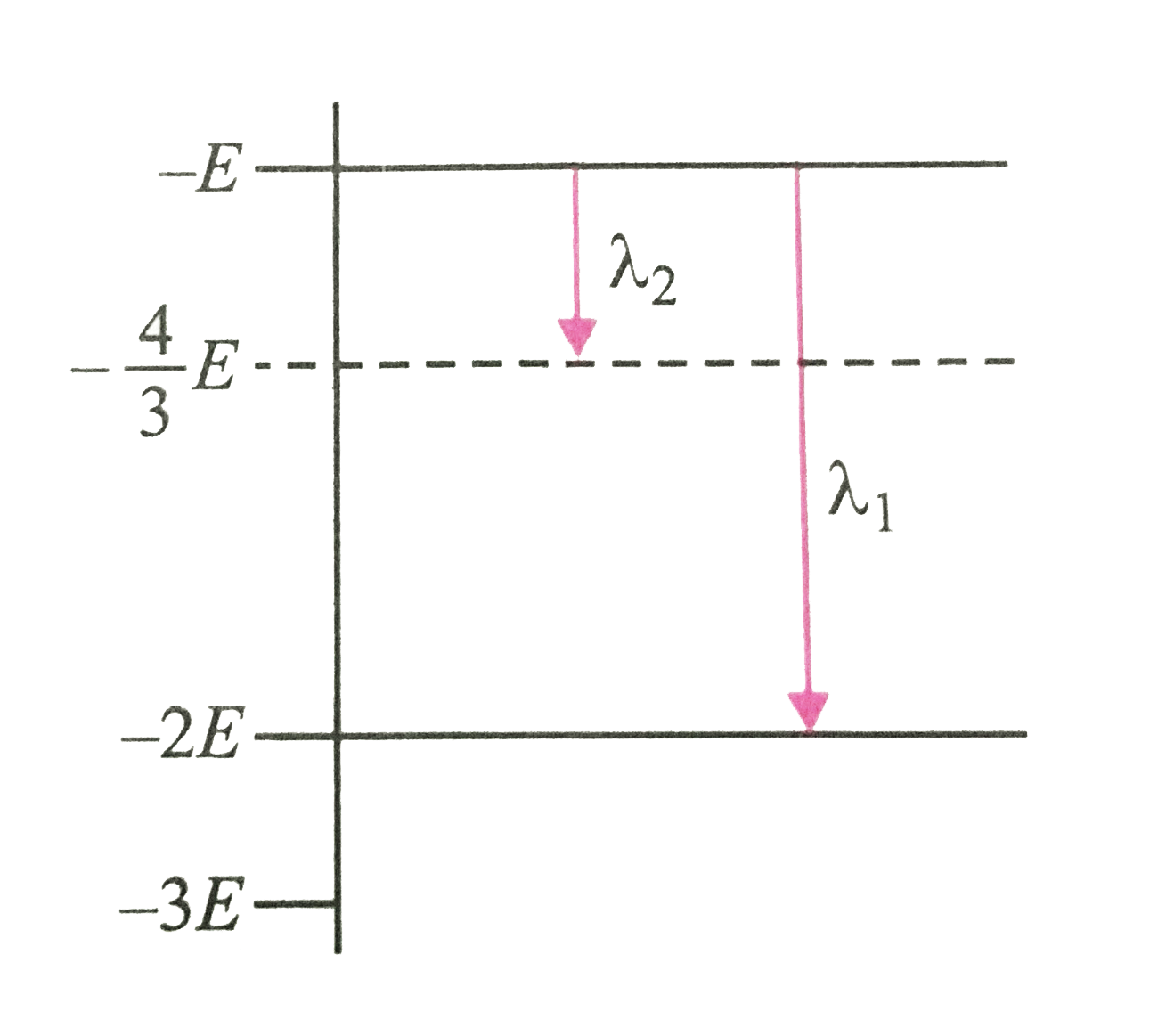
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Some energy levels of a molecule are shown in the fig. The ratio of t...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- Some energy levels of a molecule are shown in the fig. The ratio of th...
Text Solution
|
- A dye absorbs a photon of wavelength lambda and re - emits the same en...
Text Solution
|
- If lambda(1), lambda(2), lambda(3) are the wavelengths of the waves gi...
Text Solution
|
- Let be the maximum kinetic energy of photoelectrons emitted by light o...
Text Solution
|
- Some energy levels of a molecule are shown in the figure. The ratio of...
Text Solution
|
- एक प्रोटॉनकी गतिज ऊर्जा एक फोटॉन की ऊर्जा E के बराबर है । यदि प्रो...
Text Solution
|
- एक अणु के कुछ ऊर्जा-स्तरों को चित्र में दिखाया गया है। तरंगदैर्घ्यों क...
Text Solution
|