A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
JEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise Physics-Section A |20 VideosJEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise Physics-Section B|10 VideosJEE MAINS 2022
JEE MAINS PREVIOUS YEAR|Exercise PHYSICS (SECTION B)|10 VideosJEE MAINS 2021
JEE MAINS PREVIOUS YEAR|Exercise Physics (Section B )|10 VideosJEE MAINS 2023 JAN ACTUAL PAPER
JEE MAINS PREVIOUS YEAR|Exercise Question|360 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAINS 2022-Physics -Section A
- An object starts moving with constant acceleration 'a'. Distance trave...
Text Solution
|
- At what temperature a gold ring of diameter 6.230 cm be heated so that...
Text Solution
|
- Two particles A and B having equal charges are placed at distance d ap...
Text Solution
|
- The speed of light in media 'A' and 'B' are 2.0 xx 10^(10) cm//s and 1...
Text Solution
|
- In the following nuclear reaction, D overset(alpha)rarr D(1) overset(b...
Text Solution
|
- The electric field at a point associated with a light wave is given by...
Text Solution
|
- A capacitor is discharging through a resistor R. Consider in time t(1)...
Text Solution
|
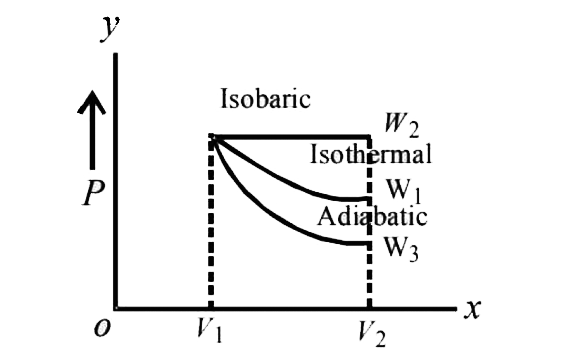
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- Two long current carrying conductors are placed parallel to each other...
Text Solution
|
- The time period of a satellite revolving around earth in a given orbit...
Text Solution
|
- The TV transmission tower at a particular station has a height of 125m...
Text Solution
|
- The motion of a simple pendulum executing S.H.M is represented by the ...
Text Solution
|
- A Vessel contain 16gm of hydrogen and 128gm of Oxygen at Standard temp...
Text Solution
|
- Given below are two statements: Statement I: The electric force chan...
Text Solution
|
- A block of mass 40kg slides over a surface, when a mass of 4kg is sus...
Text Solution
|
- In the given figure, the block of mass m is dropeed from the point 'A'...
Text Solution
|
- A block of mass M placed inside a box descends vertically with acceler...
Text Solution
|
- If the electric potential at any point (x, y, z) m in space is given b...
Text Solution
|
- Two similar cells, whether joined in series or in parallel, have the s...
Text Solution
|
- A cricketer can throw a ball to a maximum horizontal distance of 100 m...
Text Solution
|