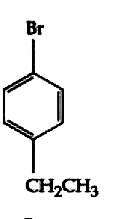
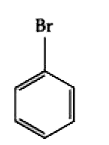
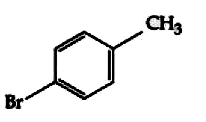
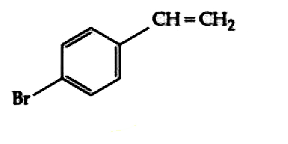
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A Aunderset((ii) CN^(-))overset((i)Cl(2)Delta)to (iii) H(2)O"/"H^(+)...
Text Solution
|
- In the given reaction CH(3)-COOHundersetunderset((iii)." "H(2)O //H^...
Text Solution
|
- CH(3)CN+H(2)Ooverset(H^(+))toA overset("Excess" Cl(2))underset(Red P)t...
Text Solution
|
- In the following sequence of reactions the products A to G are formed:...
Text Solution
|
- In the following sequence of reactions the products A to G are formed:...
Text Solution
|
- overset((i)LiAIH(4))underset((ii)H(2)O)to(A) Product (A) of above reac...
Text Solution
|
- Identify A and B in the following reactions : "Acetone" overset(2[H])r...
Text Solution
|
- Write the product of the following reaction: CH(3)-CH=CH-CH(2)CN under...
Text Solution
|
- Complete the reaction below: C Cl(4)+H(2)O overset(Delta)to
Text Solution
|