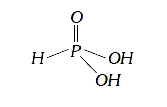
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If the molecular weight of H(3)PO(3) is M, its equivalent weight will ...
Text Solution
|
- Equivalent weight of H(3)PO(2) in a reaction is found to be half of it...
Text Solution
|
- Equivalent weight of H(3)PO(2) when it disproportionates into PH(3) an...
Text Solution
|
- H(3)PO(3)overset(Delta)toH(3)PO(4)+PH(3)uarr
Text Solution
|
- Molecular weight of KBrO(3) is M. What is its equivalent weight, if th...
Text Solution
|
- If the molecular weight of H(3) PO(3) is M, its equivalent weight will...
Text Solution
|
- Equivalent weight of H(3) PO(2) is ( M rarr molecular weight)
Text Solution
|
- STATEMENT - 1 : Equivalent weight of a substance can never be greater ...
Text Solution
|
- A: In the reaction 2NaOH + H(3)PO(4) to Na(2)HPO(4) + 2H(2)O equi...
Text Solution
|