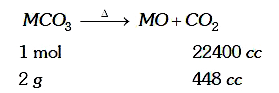A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A 100% pure sample of a divalent metal carbonate weighing 2 g on compl...
Text Solution
|
- Metal carbonates decompose on heating to give metal oxide and carbon d...
Text Solution
|
- A 100% pure sample of a divalent metal carbonate weighing 2g on comple...
Text Solution
|
- (6.023xx10^(23)xx1)/10^(20)=6023 sec=1.673 hrs. A 100% pure sample of ...
Text Solution
|
- Metal carbonates decompose on heating to give metal oxide and carbon d...
Text Solution
|
- A 100% pure sample of a divalent metal carbonate weighing 2 g on compl...
Text Solution
|
- 1g of an impure sample of magnesium carbonate (containing no thermally...
Text Solution
|
- 2.0 g of a metallic carbonate on decomposition gave 1.5g. Of metallic ...
Text Solution
|
- 1 g of an impure sample of magnesium carbonate (containing no thermall...
Text Solution
|