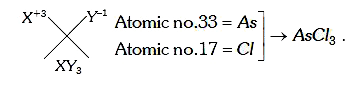A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two elements, Xand Y, have atomic numbers 33 and 17 , rspectively. The...
Text Solution
|
- Elements with atomic numbers 17 and 20 form compounds with hydrogen. W...
Text Solution
|
- Two elements, Xand Y, have atomic numbers 33 and 17 , rspectively. The...
Text Solution
|
- Element 'Y' with atomic number 3 combines with element 'A' with atomic...
Text Solution
|
- Hydrogen form compounds with elements having atomic number 9,11,12 and...
Text Solution
|
- Hydrogen forms compounds with elements having atomic numbers : 9,11,12...
Text Solution
|
- किसी तत्व A की परमाणु संख्या 12 है और दूसरे तत्व B की परमाणु संख्या 17...
Text Solution
|
- Predict the formula of stable compound formed by an element with atomi...
Text Solution
|
- What is the formula of the compound formed when an element A (atomic n...
Text Solution
|