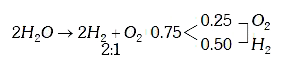A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 16.8 litre gas containing H(2) and O(2) is formed at NTP on electrolys...
Text Solution
|
- One litre of O(2) and one litre of h(2) are taken in a vessel of 2 lit...
Text Solution
|
- One litre of O(2) and one litre of h(2) are taken in a vessel of 2 lit...
Text Solution
|
- One litre of O(2) and one litre of h(2) are taken in a vessel of 2 lit...
Text Solution
|
- One litre of O(2) and one litre of h(2) are taken in a vessel of 2 lit...
Text Solution
|
- A container holds 3 liter of N(2(g)) and H(2)O((l)) at 29^(@)C The prs...
Text Solution
|
- When water is electrolysed, hydrogen and oxgen gas are produced. If 1....
Text Solution
|
- A gaseous mixture contain CH(4) and C(2)H(6) in equimolecular proporti...
Text Solution
|
- In the electrolysis of an aqueous solution of NaOH, 2.8 litres of O(2)...
Text Solution
|