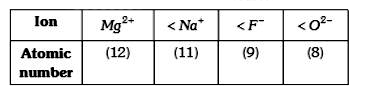
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the isoelectronic species, Na^(+),Mg^(2+),F^(-) and O^(2-). T...
Text Solution
|
- Consider the isoelectronic species, Na^(+),Mg^(2+),F^(-) and O^(2-). T...
Text Solution
|
- For Na^(+),Mg^(2+),F^(-) and O^(2-) , the correct order of increasing ...
Text Solution
|
- Consider the isoelectronic species Na^(+) , Mg^(2+) F^(-) and O^(2-). ...
Text Solution
|
- For Na^(+),Mg^(2+),F^(-) and O^(2-), the correct order of increasing i...
Text Solution
|
- Arrange the following isoelectronic species (O^(2-) , F^(-) , Na^+, Mg...
Text Solution
|
- Arrange the following isoelectronic species (O^(2-) , F^(-) , Na^+, Mg...
Text Solution
|
- Arrange the following isoelectronic species (O^(2-) , F^(-) , Na^+, Mg...
Text Solution
|
- The ionic radii of O^(2-),F^(-) , Na^(+) and Mg^(2+) are in the order ...
Text Solution
|