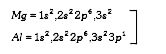A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The decreasing order of the ionization potential of the following elem...
Text Solution
|
- The decreasing order of the ionization potential of the following elem...
Text Solution
|
- The ionization potential of elements in any group decreases from top t...
Text Solution
|
- For d block elements the first ionization potential is of the order
Text Solution
|
- The decreaasing order of the ionization potential of the following ele...
Text Solution
|
- प्रथम श्रेणी के संक्रमण तत्त्वों में आयनन विभवों में वृद्धि परमाणु क्...
Text Solution
|
- The correct order of first ionization potential among following elemen...
Text Solution
|
- d-ब्लॉक के तत्वों के लिये, प्रथम आयनन विभव का क्रम है
Text Solution
|
- निम्न तत्वों के आयनन विभव का घटता हुआ क्रम है
Text Solution
|