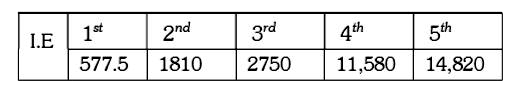
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For one of the element various successive ionization enthalpies (in 1 ...
Text Solution
|
- The first four ionization enthalpies of an element are 578 kJ mol^(-1)...
Text Solution
|
- Successive ionization enthalpies (in eV/atom) of on element are 5,8,9,...
Text Solution
|
- An element has successive ionization enthalpies as 940 (first),2080,30...
Text Solution
|
- The first and second ionization enthalpies and electron gain enthalpy ...
Text Solution
|
- If the ionization enthalpy and electron gain enthalpy of an element ar...
Text Solution
|
- Various successive ionization enthalpies (in kJ mol^(-1)) of an elemen...
Text Solution
|
- For one of the element various successive ionization ethalpies ( in...
Text Solution
|
- For one of the element various successive ionization enthalpy (in kJ "...
Text Solution
|