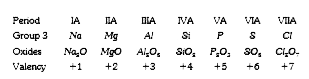A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In any period, the valency of an element with respect to oxygen
Text Solution
|
- In any period, the valency of an element with respect to oxygen
Text Solution
|
- The number of valence electrons that can be present in the second elem...
Text Solution
|
- In any period of the periodic table, valency of an element with respec...
Text Solution
|
- In any period the valency of an element with respect to oxygen
Text Solution
|
- A: Elements along a period possess different chemical properties...
Text Solution
|
- What is valency of an element? How does it vary with respect to hydrog...
Text Solution
|
- Define elemental valence by taking the example of silicon and oxygen.
Text Solution
|
- The number of valence electrons present in the 12th element of any per...
Text Solution
|