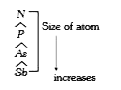A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- the pentavalence in phosphorus is more stable as compared to that of n...
Text Solution
|
- the pentavalence in phosphorus is more stable as compared to that of n...
Text Solution
|
- Nitrogen is relatively inert as compared to phosphorus. Why ?
Text Solution
|
- Explain why the alkaline earth metals react with nitrogen to form nitr...
Text Solution
|
- Why can nitrogen not show pentavalency but phosphorus?
Text Solution
|
- Pentavalence in phosphorous is more stable when compared to that of ni...
Text Solution
|
- Nitrogen and phosphorus are elements in the same group but property of...
Text Solution
|
- Among the 15^(th) group elements, as we move from nitrogen to bismuth...
Text Solution
|
- The kinetically more stable alltrope of phosphorus is
Text Solution
|