A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
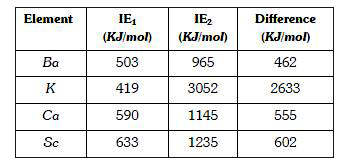
- The element having greatest difference between its first and second io...
Text Solution
|
- किसी तत्व की द्वितीय आयनन ऊर्जा इसकी प्रथम आयनन ऊर्जा की अपेक्षा अधिक ...
Text Solution
|
- एक तत्व की द्वितीय तथा तृतीय आयनन ऊर्जाओं के मानो में अंतर् बहुत अधिक ...
Text Solution
|
- समूह के 13 तत्वों की द्वितीय तथा तृतीय आयनन ऊर्जाएं उनकी प्रथम आयनन ऊर...
Text Solution
|
- किसी दिये गये तत्व की द्वितीय आयनन ऊर्जा प्रथम आयनन ऊर्र्जा से सदैव अध...
Text Solution
|
- Second ionization energy of an element is higher than its fir...
Text Solution
|
- Second ionization energy of an element is higher than its firs...
Text Solution
|
- The element having greatest difference between its first and second io...
Text Solution
|
- किसी दिए गए तत्व की द्वितीय आयनन ऊर्जा प्रथम आयनन ऊर्जा से सदैव कम हो...
Text Solution
|