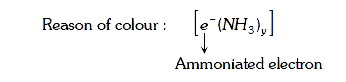A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sodium metal on dissolution in liquid ammonia gives a deep blue soluti...
Text Solution
|
- Sodium metal dissolves in liquid ammonia and forms a deep blue solutio...
Text Solution
|
- When sodium is dissolved in liquid ammonia, a solution of deep blue co...
Text Solution
|
- When sodium is dissolved in liquid ammonia , a solution of deep blue c...
Text Solution
|
- द्रव अमोनिया में सोडियम धातु नीला रंग देती है यह .............. के कार...
Text Solution
|
- Sodium metal on dissolution in liquid ammonia gives a deep blue soluti...
Text Solution
|
- A solution of sodium in liquid ammonia is blue in colour due to:
Text Solution
|
- When sodium is dissolved in liquid ammonia, a solution of deep blue co...
Text Solution
|
- When sodium (Na) metal is dissolved in liquid ammonia ("NH"(3)), it im...
Text Solution
|