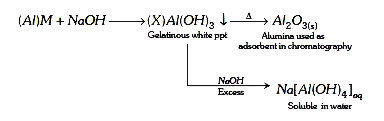A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When metal ‘M’ is treated with NaOH, a white gelatinous precipitate ‘X...
Text Solution
|
- When metal X is treated with sodium hydroxide, a white precipitate (A)...
Text Solution
|
- When metal ‘M’ is treated with NaOH, a white gelatinous precipitate ‘X...
Text Solution
|
- When metal 'M' is treated with NaoH, a white gelatinous precipitate 'X...
Text Solution
|
- When metal X is treated with sodium hydroxide, a white precipitate (A)...
Text Solution
|
- When metal 'M' is treated with NaOH, a white gelatinous precipitate ...
Text Solution
|
- 27g of Al was treated with NaOH solution when a white gelatinous preci...
Text Solution
|
- जब एक धातु M को NaOH के साथ अभिक्रिया किया जाता है तो एक सफेद जिलेटिनस...
Text Solution
|
- When metal X is treated with sodium hydroxide, a white precipitate (A)...
Text Solution
|