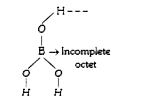A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule
Text Solution
|
- Boric acid is an acid because its molecule -
Text Solution
|
- बोरिक अम्ल एक अम्ल है, क्योंकि इसके अणु
Text Solution
|