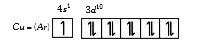A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Total number of unpaired electrons in an atom of atomic number 29 is
Text Solution
|
- Total Number of unpaired electrons in d- orbitals of an atom element o...
Text Solution
|
- The number of unpaired electrons in the scandium atom is
Text Solution
|
- The number of unpaired electrons in the scandium atom is
Text Solution
|
- Total number of unpaired electrons in an atom of atomic number 29 is
Text Solution
|
- Find the number of unpaired electrons present in phosphorus (atomic no...
Text Solution
|
- The number of unpaired electrons in magnesium atom is
Text Solution
|
- The number of unpaired electrons in carbon atom is -
Text Solution
|
- The number of unpaired electrons in carbon atom is -
Text Solution
|