A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of valence electrons in an atom with electronic configurati...
Text Solution
|
- The number of valence electrons in an atom with the configuration 1s^(...
Text Solution
|
- The number of valence electrons in an atom with the configuration 1s^(...
Text Solution
|
- An element has the electronic configuration 1s^2 , 2s^2 2p^6 , 3s^2 3p...
Text Solution
|
- Name the elements that correspond to the given electronic configuratio...
Text Solution
|
- एक तत्व का इलेक्ट्रॉनिक विन्यास 1s^(2), 2s^(2)2p^(6), 3s^(2), 3p^(1) ह...
Text Solution
|
- एक तत्व का इलेक्ट्रॉनिक विन्यास 1s^(2)2s^(2)2p^(6)3s^(2)3p^(3) है। इसक...
Text Solution
|
- किसी तत्व का इलेक्ट्रॉनिक विन्यास 1s^(2),2s^(2),2p^(6),3s^(2),3p^(2) ...
Text Solution
|
- The electron configurations of atoms A and B are: 1s^(2) 2s^(2) 3p^(6)...
Text Solution
|
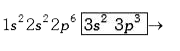 Valence shell `e^(-)` i.e. two in s-subshell and three in p-subshell
Valence shell `e^(-)` i.e. two in s-subshell and three in p-subshell