A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following about an electron occupying the 1s orbital ...
Text Solution
|
- Which one of the following about an electron occupying the 1s orbital ...
Text Solution
|
- In terms of Bohr radius a(0) , the radius of the second Bohr orbit of ...
Text Solution
|
- If the de Broglie wavelength of the electron in n^(th) Bohr orbit in a...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- एक हाइड्रोजन परमाणु में द्वितीय बोर कक्षा में इलेक्ट्रॉन की गतिज ऊर्जा...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- In hydrogen atom,the de Broglie wavelength of an electron in the secon...
Text Solution
|
- In terms of Bohr radius a(0), the radius of the third Bohr orbit of a ...
Text Solution
|
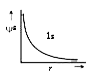 `Psi^2` is maximum at nucleus
`Psi^2` is maximum at nucleus 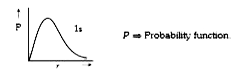 P `implies` Probability function
P `implies` Probability function