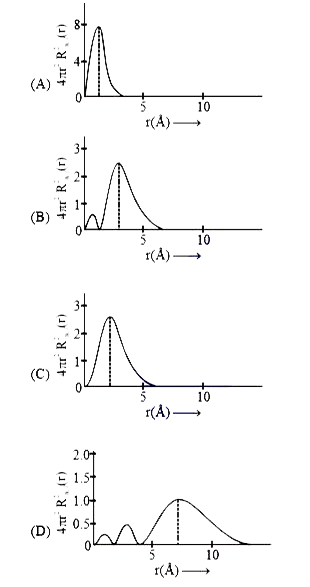
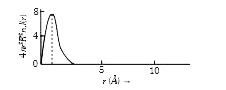
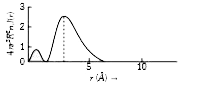
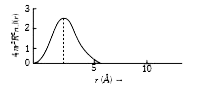
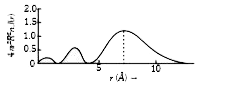
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The plots of radial distribution function for various orbitals of hydr...
Text Solution
|
- OPERATOR FORM SCHRODINGER WAVE EQUATION,PLOT OF RADIAL WAVE FUNCTION '...
Text Solution
|
- In a hydrogen atom, which orbital is higher in energy than a 3s-orbita...
Text Solution
|
- Wave function of an orbital is plotted against the distance from nucle...
Text Solution
|
- Plot of Maxwell's distribution of velocities is given below: which of ...
Text Solution
|
- In the plots of radial distribution function for the hydrogen 3s orbit...
Text Solution
|
- In a hydrogen atom, which orbital is higher in energy than 3s-orbital ...
Text Solution
|
- Which of the following is correct radial probability distribution curv...
Text Solution
|
- Wave function of an orbital is plotted against the distance from nucle...
Text Solution
|