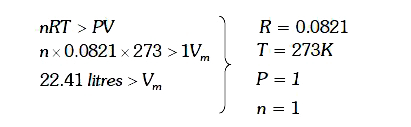A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compressibility of a gas is less than unity at STP .
Text Solution
|
- The compressibility factor of gases is less than unity at STP . Theref...
Text Solution
|
- The compressibility of a gas is less than unity at STP .
Text Solution
|
- The compressibility of a gas is less than unity at S.T.P
Text Solution
|
- The compressibility of a gas is less than unity at N.T.P. Therefore.
Text Solution
|
- STP पर गैस की संपीड्यता इकाई से कम होती है | अतः
Text Solution
|
- The compressibility of a gas is less than unity at STP. Therefore,
Text Solution
|
- The compressibility factor of gases is less than unity at STP. Therefo...
Text Solution
|
- STP पर एक गैस की संपीडयता (compressibility) इकाई से कम है अतः
Text Solution
|