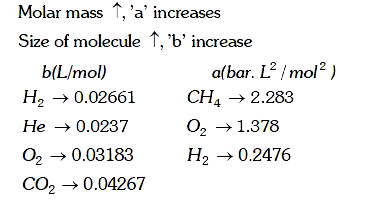A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For real gases, van der Waals' equation is written as (P+(an^(2))/(V...
Text Solution
|
- Considering van der Waals equation of state for a real gas (P+n^(2)a//...
Text Solution
|
- For real gases, van der Waals' equation is written as (P+(an^(2))/(V...
Text Solution
|
- For real gases, van der Waal's equation is written as [p + (an^(2))/(V...
Text Solution
|
- For real gases the relation between p, V and T is given by c=van der W...
Text Solution
|
- For real gases the relation between p, V and T is given by van der Waa...
Text Solution
|
- For real gases, van der Waals equation is written as (p+(an^(2))/(V^(2...
Text Solution
|
- वास्तविक गैसों के लिए वाण्डरवाल समीकरण इस प्रकार लिखा जाता है, (P + ...
Text Solution
|
- Set-I: O(2),CO(2),H(2) and He, Set-II: CH(4),O(2) and H(2) . The gases...
Text Solution
|