A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
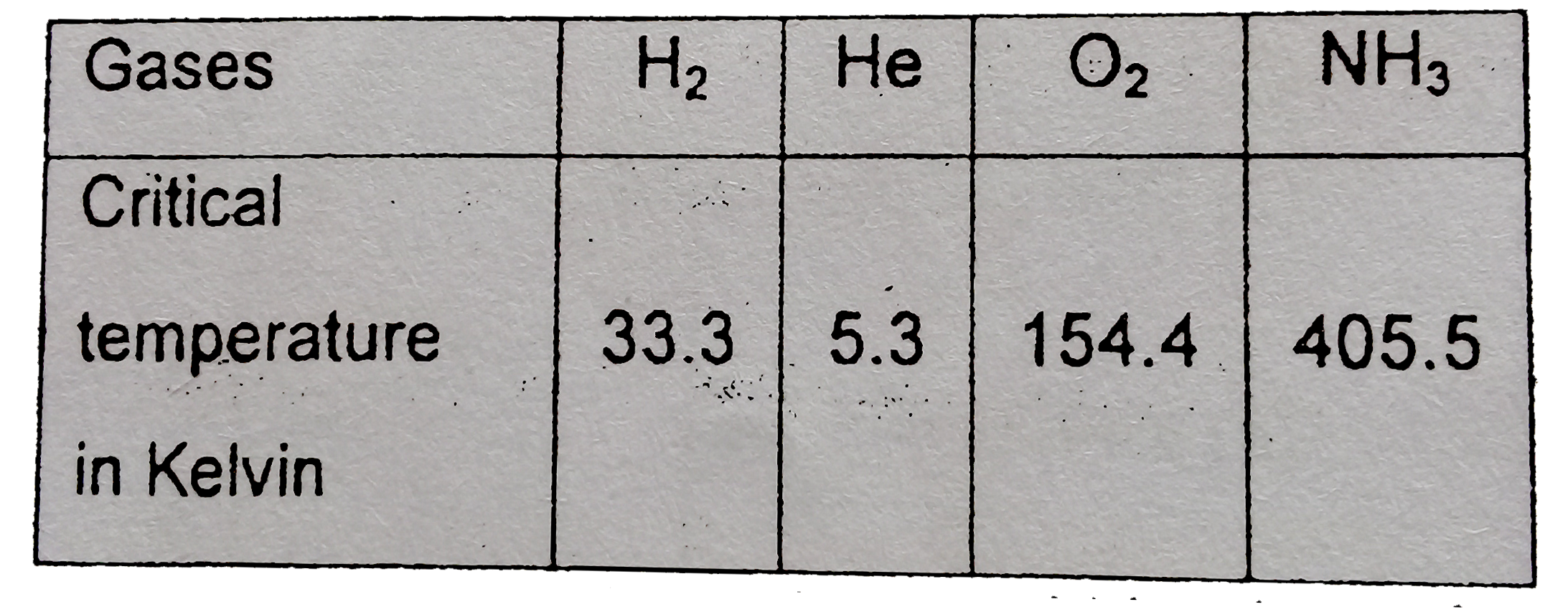
- Gases possess characteristic critical temperature which depends upon t...
Text Solution
|
- Gases possess characteristic critical temperature which depends upon t...
Text Solution
|
- {:("Gas",H(2),He,O(2),N(2)),("Critical temp. ink",33.2,5.3,154.3,126):...
Text Solution
|
- Gases possess characteristic critical temperature which depends upon t...
Text Solution
|
- Gases posses characteristic critical temperature which depends upon th...
Text Solution
|
- Gases possess characteristic critical temperature which depends upon t...
Text Solution
|
- Gases posses characteristic critical temperature which depends upon th...
Text Solution
|
- The critical temperature of NH(3),CO(2) and O(2) gases are 405.6K, 304...
Text Solution
|
- Gases possess characteristic critical temperature which depends upon t...
Text Solution
|