Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
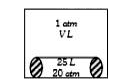
- Inside a drum of V'L a sealed glass tube of volume 25L containing n in...
Text Solution
|
- A cylindrical drum is partially filled with liquid.A cross section of ...
Text Solution
|
- A tightly sealed 25.0 litre acetone drum was found to have 15.4 litre ...
Text Solution
|
- Inside a drum of V'L a sealed glass tube of volume 25L containing n in...
Text Solution
|
- Ear drum is
Text Solution
|
- Drums of oil are carried in a truck. If a constant accleration is appl...
Text Solution
|
- A drum full of rice weighs 40(1)/(6)kg. If the empty drum weighs 13(3)...
Text Solution
|
- Pressure inside the tympanic cavity of middle ear is kept equal to tha...
Text Solution
|
- 21 সেমি. দৈর্ঘ্যর ব্যাসার্ধ ও 21 সেমি. উচ্চতাবিশিষ্ট একটি লম্ব বৃত্তাক...
Text Solution
|