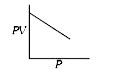
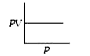
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following is the correct PV vs P plot at constant tem...
Text Solution
|
- STATEMENT-1 : Plot of P vs 1/V (volume) is a straight line for an idea...
Text Solution
|
- एक आदर्श गैस pV^(2) = नियतांक, नियम का पालन करती है | गैस का प्रारम्भि...
Text Solution
|
- A plot of P vs T for a given mass of gas at constant volume is a strai...
Text Solution
|
- What is the nature of graph of PV versus P for a given mass of a gas a...
Text Solution
|
- Plot density vs pressure for a fixed mass of an ideal gas at a constan...
Text Solution
|
- Why is the PV vs P plot for an ideal gas at a given temperature parall...
Text Solution
|
- for a given mass of an ideal gas at constant temperature draw and expl...
Text Solution
|
- The ideal gas equation for 1 mol of ideal gas is PV = nRT graph betw...
Text Solution
|