A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
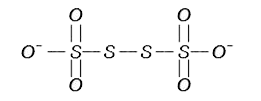
- The oxidation states of S atoms in S4O6^(2-) from left to right respe...
Text Solution
|
- The oxidation states of S atoms in S(2)O(6)^(2-) from left to right re...
Text Solution
|
- The oxidation states of S atoms in S(4)O(6)^(2-) from left to right re...
Text Solution
|
- What happens to the oxidation state of the elements of the second movi...
Text Solution
|
- The oxidation states of S atom in S(4)O(6)O^(2-) from left to right re...
Text Solution
|
- S(4)O(6)^(2-) में S परमाणुओं की ऑक्सीकरण अवस्था बायें से दायें क्रमशः ...
Text Solution
|
- The oxidation states of S atom in S(4)O(6)^(2-) from left to right res...
Text Solution
|
- Basic nature of the oxides of a period from left to right
Text Solution
|
- Basic nature of the oxides of a period from left to right
Text Solution
|