A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
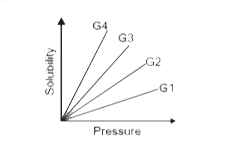
- The variation of solubility of four different gases (G1, G2, etc.) in ...
Text Solution
|
- Assertion : On reducing the value of a gas at constant temperature, th...
Text Solution
|
- According to William Henry,the solubility of a gas in liquid depends o...
Text Solution
|
- A plot of volume (V) versus temperature (T) for a gas at constant pres...
Text Solution
|
- Molar solubility of helium, nitrogen and oxygen are plotted against pa...
Text Solution
|
- Which one of the following gases has the lowest value of Henry law con...
Text Solution
|
- Assertion. Greater the value of Henry's constant of a gas in a particu...
Text Solution
|
- when pressure is increaed at constant temperature the solubility of ga...
Text Solution
|
- समान ताप पर गैस (A) अपेक्षाकृत गैस (B) के तुलना में जल में अधिक घुलनश...
Text Solution
|