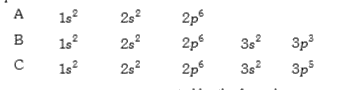A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The electronic configuration of the elements A, B and C are given belo...
Text Solution
|
- Electronic configurations of four elements A, B, C and D are given bel...
Text Solution
|
- The molecular formula of the compound formed from B and C will be :
Text Solution
|
- The electronic configuration ofhte elements. A, B and C are given belo...
Text Solution
|
- The electronic configurations of three elements, A,B are C are given b...
Text Solution
|
- How is the compound formed when the element A having electronic config...
Text Solution
|
- Given below is the electronic configuration of elements A ,B ,C...
Text Solution
|
- A,B,C,D మూలకాల ఎలక్ట్రాన్ విన్యాసాలను క్రింద ఇవ్వబడమైనది. వీటి ఆధారంగా...
Text Solution
|
- The electronic configuration ofhte elements. A, B and C are given belo...
Text Solution
|