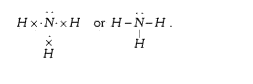A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Number of electrons in the valence orbit of nitrogen in an ammonia mol...
Text Solution
|
- The total number of electrons in a nitrogen atom is 7. Find the number...
Text Solution
|
- Number of electrons in the valence orbit of nitrogen in an ammonia mol...
Text Solution
|
- The valency of nitrogen in ammonia (NH(3)) is
Text Solution
|
- The number of electrons in the valence shell of nitrogen in an amine i...
Text Solution
|
- Valencies of nitrogen and hydrogen and 3 and 1 respectively. The formu...
Text Solution
|
- The number of electrons in the valence shell of the central atom of a ...
Text Solution
|
- नाइट्रोजन में संयोजी इलेक्ट्रॉन की संख्या है
Text Solution
|
- కక్ష్యలో గల ఎలక్ట్రాన్లను వేలన్సీ ఎలక్ట్రాన్లు అంటారు.
Text Solution
|