A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of lone pair of electrons on the central atoms of H2O, SnCl...
Text Solution
|
- In XeF2, XeF4 amnd XeF6 , the number of lone pair of electrons on Xe a...
Text Solution
|
- The number of lone pairs on central atom in XeF2, XeF4 and XeF6 are:
Text Solution
|
- In the structure of ClF(3), the number of lone pairs of electrons on c...
Text Solution
|
- The number of lone pair of electrons in central atom of CIF5
Text Solution
|
- The ratio of number of lone pairs on central atom in ammonia, Water an...
Text Solution
|
- H2O , SnCl 2 , PCl3 And XeF2 The number of solitary electron pairs on ...
Text Solution
|
- H2O , SnCl2 , PCl3 और XeF2 के केन्द्रिय परमाणु पर इलेक्ट्रॉन्स के एकाक...
Text Solution
|
- In which of the following compounds central atom has same number of lo...
Text Solution
|
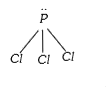 `rArr ` No. of lone pairs = 1
`rArr ` No. of lone pairs = 1