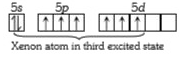
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In XeF(6), oxidation state and of hybridisation of Xe and shape of the...
Text Solution
|
- In XeO(3) and XeF(6) the oxidation state of Xe is
Text Solution
|
- The oxidation state of Xe in XeF(6) is
Text Solution
|
- In XeF(6) , oxidation state and of hybridisation of Xe and shape of th...
Text Solution
|
- XeF(6) में Xe की ऑक्सीकरण अवस्था ........... तथा संस्करण अवस्था .........
Text Solution
|
- Number of lone pairs of electrons on Xe atoms XeF(2),XeF(4) and XeF(6...
Text Solution
|
- Number of lone pairs of electrons on Xe atoms in XeF(2), XeF(4), XeF(6...
Text Solution
|
- Mention the state of hybridisation of Xe atom in each of the following...
Text Solution
|
- Mention the state of hybridisation of Xe atom in each of the following...
Text Solution
|