A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
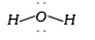
- How many electrons pairs are present in valence shell of oxygen in wat...
Text Solution
|
- How many electrons are present in the valence shell of P in PCl3 ?
Text Solution
|
- How many line paire of electrons are present in outer shell of Cl^- ?
Text Solution
|
- How many electrons are present in the valence shells of the central at...
Text Solution
|
- How many electrons pairs are present in valence shell of oxygen in wat...
Text Solution
|
- How many electron pairs are present in valence shell of oxygen in wt...
Text Solution
|
- How many molecules of water and oxygen atoms are present in 0.9g of wa...
Text Solution
|
- How many unpaired electrons are present in oxygen molecule ?
Text Solution
|
- जल के अणु के मध्य परमाणु ऑक्सीजन पर दो एकाकी इलेक्ट्रॉन युग्म उपस्थित ...
Text Solution
|