A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
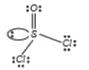
- In SOCl(2), the Cl-S-Cl and Cl-S-O bond angles are
Text Solution
|
- Cl-P-Cl bond angles in PCl5 molecule are
Text Solution
|
- In structure Cl-overset(O)overset(||)underset(O)underset(||)(S)-Cl and...
Text Solution
|
- Cl-C-Cl bond angle in C Cl(4) is .
Text Solution
|
- Cl-P-Cl bond angles in PCl5 molecule are
Text Solution
|
- Bond between Cl-Cl is .
Text Solution
|
- In SOCl2, the Cl-S-Cl and Cl-S-O bond angles are
Text Solution
|
- Cl-P-Cl bond angles in PCl(5) molecule are
Text Solution
|
- In SOCl(2) , the Cl-S-Cl and Cl-S-O bond angles are
Text Solution
|