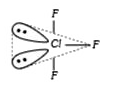
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The shapes of molecules of BF3, NH3 and ClF3 are
Text Solution
|
- Shape of ClF3 is
Text Solution
|
- Among the molecules, SO2 , SF4 , ClF3 . BrF5 and XeF4 which of the fol...
Text Solution
|
- The shapes of molecules of BF3 , NH3 and ClF3 are
Text Solution
|
- The correct increasing bond angle among BF3 , PF3 and ClF3 follows the...
Text Solution
|
- Comment on the shapes of BF3 molecule and BF4^(-) ion.
Text Solution
|
- ClF3 अणु की आकृति है
Text Solution
|
- Give the shape of ClF3 .
Text Solution
|
- BF3 and NH3 are ….. In the reaction BF3+NH3 to BF3 larr NH3 .
Text Solution
|