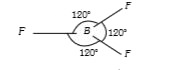
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following molecules has trigonal planar geometry ?
Text Solution
|
- Which of the following molecules planer planar geometry?
Text Solution
|
- Which molecule has trigonal planar geometry ?
Text Solution
|
- Which of the following molecule has trigonal planner geometry?
Text Solution
|
- Which of the molecules has trigonal bipyramidal geometry with bond ang...
Text Solution
|
- Which of the following will be planar trigonal ?
Text Solution
|
- Which of the following will be planar trigonal ?
Text Solution
|
- Formamide has the structure HC(O)NH(2) . Which atoms in formamide have...
Text Solution
|
- Which reaction forms a product with a trigonal planar geometry?
Text Solution
|