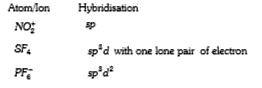A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the correct mode of hybridization of the central atom in the f...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- What is the correct mode of hybridization of the central atom in the f...
Text Solution
|
- NO(3)^(-) आयन के केन्द्रीय नाइट्रोजन परमाणु पर संकरण है :
Text Solution
|
- The hybridization states of the central atom in the complexes [Fe(CN)(...
Text Solution
|
- What is the hybrid state of the central atom in each of following? BF(...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Find the total number of polar molecules SF(4),PCl(5),PCl(3)F(2),SF(6)...
Text Solution
|
- Find the total number of compounds or ions having different bond lengt...
Text Solution
|