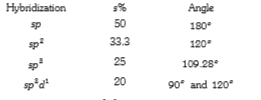A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- As the s-character of hybridisation orbital increases, the bond angle
Text Solution
|
- Angle between two hybridised orbital is 105^(@) and hence the percenta...
Text Solution
|
- As the s-character of hybridisation orbital increases, the bond angle
Text Solution
|
- Assertion: Hybridisation influences the bond length and bond enthalpy ...
Text Solution
|
- Which is correct statement ? As the s-character of a hybrid orbital de...
Text Solution
|
- According to hybridisation theory, the % s-character in sp, sp^(2) and...
Text Solution
|
- As the s-character of hybridisation orbital increase, the bond angle
Text Solution
|
- The change in bond angle as the s - character of hybridized orbital de...
Text Solution
|
- Which is correct statement ? As the s-character of a hybrid orbital ...
Text Solution
|