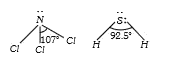A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among the compounds BF(3), NCI(3), H(2)S, SF(4) and BeCI(2)., identify...
Text Solution
|
- Among the compounds BF(3), NCI(3), H(2)S, SF(4) and BeCI(2) ., identif...
Text Solution
|
- In allene (C(3)H(4)) , the type(s) of hybridisation of the carbon atom...
Text Solution
|
- In allene (C(3)H(4)) the type(s) of hybridisation of the carbon atoms ...
Text Solution
|
- Which of the following compounds has a central atom assuming sp^(3) hy...
Text Solution
|
- Among the following compounds the one that is polar and has central at...
Text Solution
|
- In which of the following molecules / ions BF(3), NO(2)^(-), H(2) the ...
Text Solution
|
- The central S-atom of SF(6) molecule is hybridised.
Text Solution
|
- Among the coimpounds, BF(3), NH(3), H(2)O,SF(4)and BeCl(2), identify t...
Text Solution
|