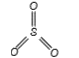
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The hybrid state of sulphur in SO3 molecule is
Text Solution
|
- What is the shape of SO3 molecule in gaseous state?
Text Solution
|
- The hybrid state of S in SO3 is similar to that of
Text Solution
|
- The hybrid state of sulphur in SO3 molecule is
Text Solution
|
- In H2S , sulphur atom is present in which hybrid state ?
Text Solution
|
- What is the hybrid state and oxidation state of sulphur in Caro's acid...
Text Solution
|
- SCl2 is the best known dihalice of sulphur, hybrid state of sulphur in...
Text Solution
|
- The hybrid state of S in SO(3) molecule is.
Text Solution
|
- The hybridisation of sulphur in SF(6) is
Text Solution
|