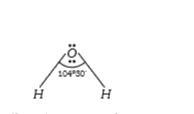
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond angle in water molecule is nearly Or Directed bonds in water ...
Text Solution
|
- Directed bond in water form an angle of
Text Solution
|
- In water molecule, the bond angle of 104.5^(@) around oxygen is accoun...
Text Solution
|
- जल के अणु में H - O - H बन्ध कोण लगभग 180° होता है।
Text Solution
|
- Why is the bond angle of water less than the bond angle of CH(4) ?
Text Solution
|
- जल के अणु में बन्ध-कोण लगभग होता है
Text Solution
|
- जल के अणु में बन्ध कोण लगभग होता है या जल के अणु में बना हुआ प्रत्यक्ष...
Text Solution
|
- Write bond angle and hybridisation type of water molecule
Text Solution
|
- Write bond angle and types of hybridization of water molecule.
Text Solution
|