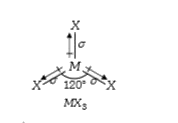A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If molecule MX3 has zero dipole moment, the sigma bonding orbitals use...
Text Solution
|
- If B-Cl bond has a dipole moment, explain why BCl(3) molecule has zero...
Text Solution
|
- If molecule MX3 has zero dipole moment, the sigma bonding orbitals use...
Text Solution
|
- The angle between the bonding orbitals of a molecules AX(3) with zero ...
Text Solution
|
- यदि एक अणु MX(3) का द्विध्रुव आघूर्ण शून्य है तब M के द्वारा प्रयुक...
Text Solution
|
- If B-Cl bond has a dipole moment, explain why BCl3 molecule has zero d...
Text Solution
|
- If B-Cl bond has a dipole moment, explain why BCl3 molecule has a zero...
Text Solution
|
- A molecule MX(3) has no dipole moment. The sigma bonding orbital used ...
Text Solution
|
- A molecule MX(3) has no dipole moment. The sigma bonding orbital used ...
Text Solution
|