A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given : Dipole moment of HCl=1.03 D Bond length =127pm Dipole mom...
Text Solution
|
- The observed dipole moment of HCl is 1.03D . If the bond length of HCL...
Text Solution
|
- HCl का प्रतिशत आयनिक लक्षण ज्ञात करो । यदि HCl के द्विध्रुव आघूर्ण का ...
Text Solution
|
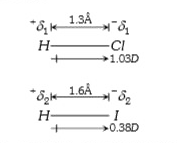
- Given : Dipole moment of HCl=1.03 D Bond length of HI=0.38D Bond...
Text Solution
|
- Calculate the ratio of partial positive charge on H-atom in HCl to tha...
Text Solution
|
- The observed dipole moment of HI is 0.38D. Calculate the percentage io...
Text Solution
|
- Dipole moment of HCl = 1.03 D, HI-0.38 D. Bond length of HCI-1.3 A^(@)...
Text Solution
|
- The bond length of HCl molecule is 1.275 A^(@) and its dipole moment i...
Text Solution
|
- Calculate the percent ionic character of HCl. Given that the observed ...
Text Solution
|